|
||||
In an article published recently in the journal Glia, we discuss some experiments we have performed recently that reveal some new ideas about Aicardi-Goutières syndrome (AGS). The article is called "Astrocytes produce interferon-alpha and CXCL10, but not IL-6 or CXCL8, in Aicardi-Goutières syndrome" and is written by Jane van Heteren, Flore Rozenberg, Eleonora Aronica, Dirk Troost, Pierre Lebon, and Taco Kuijpers, researchers from the Academic Medical Center in Amsterdam, the Netherlands and the Hospital St. Vincent de Paul/Cochin in Paris, France. Everyone familiar with AGS is aware that interferon-alpha is increased in patients, that it is higher in the cerebrospinal fluid than in the blood serum, and that it decreases as the children get older. Little is known, however, about other factors that might be increased in AGS patients. We therefore measured a panel of 30 different cytokines, chemokines and growth factors in the cerebrospinal fluid (csf) and serum of AGS patients. All of these factors are proteins that are made by cells in our bodies to send out as a signal into the circulation. Depending on the protein, the signal could be that there is a virus infection and that white blood cells should come to fight it off. Or the signal could be that there is damage to tissue it needs to be repaired. In addition to the samples from AGS patients, we measured sera and csf from patients with viral infection. We had some patients with an enterovirus infection and others with a herpes virus infection. Both of these viruses are known to infect the brain and patients with these infections often have symptoms similar to those of AGS. We also measured sera and csf from healthy children for comparison. We found with these measurements that some of the factors are produced in both AGS and in virus infection. As was already known, we found interferon-alpha increased in the csf of both AGS patients and patients with viral infection. In addition, we several other proteins increased in the csf of both groups that send a signal to certain types of white blood cells that they need to come to the brain. These factors may be responsible for the increased number of white blood cells seen in the csf of AGS patients. We confirmed that the pattern of expression for all of these proteins is the same. They are all generally higher in the csf than in serum, implying that they are produced in the brain. In AGS, the levels of all of these proteins decrease as the patients get older, but in virus infection, the levels are high at all ages. Besides the factors that are the same in AGS and viral infection, we found some factors that are different. There are several proteins that are increased in the csf of virally infected patients, but not in AGS patients. Because the clinical symptoms of AGS can be so similar to those of viral infection, it is normally difficult to distinguish these two diseases. Diagnosis is a time-consuming and uncertain process. With the differences we have found, distinguishing these two diseases can be done quickly, without having to individually exclude every possible viral infection. The differences that we found in expression of cytokines imply that the pathways that are affected by the genetic mutation(s) that cause AGS are different from the pathways that are turned on by viral infection. The genetic mutation does not simply mimic a viral infection. These specific differences can help us to better understand the pathophysiology of AGS. One other question that we sought to answer with these experiments is which cells are responsible for producing the cytokines, chemokines and growth factors that we see elevated in the csf of AGS patients. We therefore obtained brain material that in the past had been collected at atopsy of two patients with AGS and two patients with herpes encephalitis. We used a technique called immunohistochemistry. Basically, we cut the material into very thin sections which we can study under the microscope. Then we used antibodies that have a color attached to them. Some of these antibodies stick to the cytokines or chemokines that we know are increased in AGS, such as interferon-alpha and give them a certain color. Then we use antibodies with a different color that stick to certain proteins that allow us to recognize different cell types, like blood cells or brain cells. In this way we were found that the cell type that produces the cytokines and chemokines in AGS and in herpes encephalitis are astrocytes. Astrocytes are star-shaped cells that make up about 40% of our brains. The fact that astrocytes are producing the cytokines in AGS makes sense in a way. There is a researcher named Iain Campbell in Australia who has made a mouse that produces interferon-alpha specifically in astrocytes, and these mice have symptoms very similar to AGS patients. The fact that a brain cell, and not a blood cell, is producing the cytokines also explains why the levels of these cytokines are higher in csf than in serum. These findings change our view of AGS. Previously, it was thought that blood cells would come to the brain and that these blood cells would produce the cytokines that we see in the csf of AGS patients. A certain type of white blood cell called a plasmacytoid dendritic cell can produce large quantities of interferon-alpha. This cell was thought to produce the interferon-alpha in AGS. Now we know that a brain cell produces the cytokines and these cytokines attract the blood cells as a secondary effect. In conclusion we now have a way to distinguish AGS from viral infection, which will help in the diagnosis of the disease. We know that a brain cell, not a blood cell, is responsible for producing cytokines in AGS.
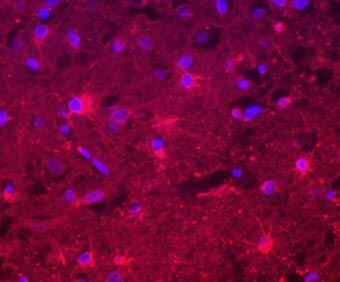
The interferon-alpha in a section from the brain of an AGS patient is labeled red. The blue spots are the cell nuclei. The cells producing the interferon-alpha are star-shaped astrocytes. |
||