| |
We have recently published an article in Neuropediatrics with the first results from our research on the cause of Aicardi-Goutières syndrome (AGS). The article is called "Plasmacytoid dendritic cells and interferon-alpha in Aicardi-Goutières syndrome" by Jane van Heteren, Marjo van der Knaap, BweeTien Poll The, and Taco Kuijpers. We summarize here the most important points from the article.
AGS is characterized by a prolonged increase in interferon-alpha (IFNα) expression. This IFNα may play an important role in the clinical picture that we see in patients. We believe this to be true because a mouse that was specifically designed to have IFNα produced in the brain shows many of the same symptoms as AGS patients, such as calcium deposits in the brain, seizures and an accumulation of white blood cells in the cerebrospinal fluid.
We wanted to compare AGS to other diseases that are also characterized by a prolonged increase in IFNα. SLE, or lupus, is one of these diseases. SLE patients show some symptoms in common with AGS patients, such as chilblains or "winter toe." Especially the pediatric form of SLE has sometimes been confused with AGS. One important difference to keep in mind, however, is that the IFNα in SLE is not higher in the brain than in blood, as it is in AGS.
In SLE, it is fairly well known how the IFNα gets there. In all people, there are a certain number of cells in our bodies that die, and these cells are normally cleaned up by our white blood cells and there is no problem. In SLE, the cells are not cleaned up properly, and pieces cells are left behind, such as pieces of DNA and RNA. SLE patients develop antibodies against these DNA and RNA pieces. These antibodies are called ANA for antinuclear antibodies and are used in the diagnosis of SLE.
ANA bind to the pieces of DNA or RNA and form immune complexes. These complexes activate certain white blood cells called plasmacytoid dendritic cells (pDCs), by binding to certain receptors called TLR7 (for RNA) and TLR9 (for DNA). In this way, these white blood cells are fooled into thinking that there is an infection and they make IFNα. Because the pDCs are continuously stimulated, there is a decrease in the number of these cells in circulating blood.
We wanted to test whether or not these same processes are operating in AGS patients. We first tested whether or not AGS patients have ANA (antibodies to DNA or RNA). We found that some of them do have these antibodies, but the frequency of patients having these ANA is not much higher than the frequency in the healthy population. ANA are therefore not characteristic of AGS, as they are for SLE.
We also asked whether or not AGS patients have any soluble factors in their blood (or cerebrospinal fluid) which could activate pDCs. We do find these factors in the blood of SLE patients, but we do not find them in AGS patients.
Thirdly, we asked whether or not AGS patients have reduced numbers of pDCs in their circulating blood. We found no differences in numbers of these white blood cells compared to healthy donors.
These results all indicate that the cause of AGS is very different from the cause of SLE. If is unlikely that pDCs are the source of IFNα in AGS patients.
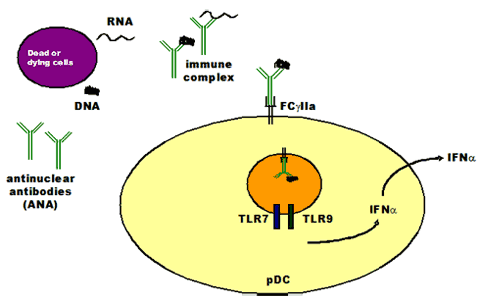
Mechanism of IFNα production in SLE but not in AGS
|
|
